The UKKA Laboratory Scientists Research Committee (UKKA-LSRC)
A new home for basic science research in kidney disease
This May section celebrating the 75th Anniversary of the UK Kidney Association (UKKA) focuses on basic science and its contribution to our understanding and treatment of kidney disease.
To take forward the basic science work of the UKKA, in 2021 the UKKA Laboratory Scientists Research Committee (UKKA-LSRC) was formed. The original core group was inaugural UKKA-LSRC chair Prof Timothy Bowen (Cardiff University), current chair Dr Laura Denby (University of Edinburgh), Prof Gavin Welsh (University of Bristol) and Dr Mark Dockrell (St Helier Hospital, London), derived largely from previous entity the Renal Association’s Renal Scientists Working Group.
The UKKA-LSRC was formed to create an inclusive association of non-clinical scientist, multi-professional team and clinician scientist UKKA members interested in the science of kidney disease, including representation from Kidney Research UK. The inaugural face-to-face meeting of the UKKA-LSRC took place at UK Kidney Week 2022 (UKKW 2022).
The story so far
UKKW 2022 featured a survey to canvass members’ opinions on research involvement and access to research opportunities. In response to these survey data, the UKKA Stewart Cameron Award* was launched at UKKW 2024 to recognise outstanding contributions to the basic scientific understanding of kidney disease. Also instituted for the first time at UKKW 2024, one UK Kidney Week cochair position was created for a basic renal scientist.
*For enquires about the Stewart Cameron Award and nominations, please contact ukka@ukkidney.org
Building research networks to improve patient outcomes
In addition to representing UKKA members, the UKKA-LSRC also seeks to grow a broader Kidney Scientists’ Research Network. The aim of this Network will be to build an extended collective, centred on kidney scientists but including members from other research fields with an interest in renal research.
One area of common ground via which this larger assemblage might expand is the omics technologies of genomics, metabolomics, proteomics, transcriptomics and exposomics.
The kidney is particularly well suited to transcriptomic analysis using single-cell and single-nucleus RNA sequencing (scRNA-seq, snRNA-seq) approaches. These techniques offer the opportunity to analyse the kidney at much higher resolution than previous methods. In the kidney, each nephron is composed of glomerulus, proximal and distal convoluted tubules, loop of Henle and collecting duct. Cells from these discrete functional regions have different morphological and functional phenotypes, and they can be separated by differences in their transcriptional profiles (e.g., see Figure 1). To date, analyses using scRNA-seq and snRNA-seq have already enriched our understanding of kidney biology in health and disease, and several UK kidney disease research centres have made significant contributions.
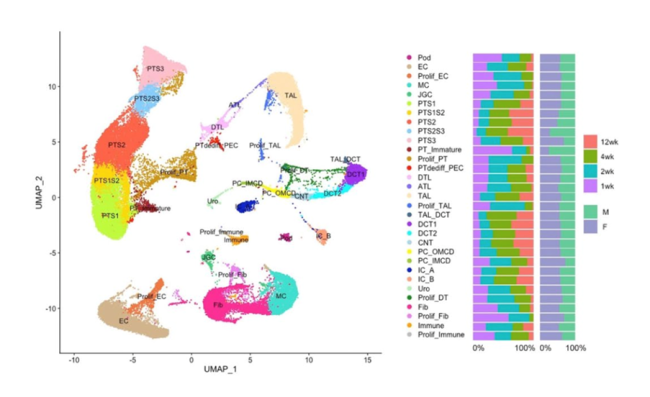
Figure 1. Single nuclear RNA sequencing of mouse kidneys
Adapted from Lu et al. (2024) Sex-specific proximal tubular cell differentiation pathways identified by single-nucleus RNA sequencing. Scientific Reports 14:24041. doi: 10.1038/s41598-024-73102-7. Licensed under CC-BY-4.0.
Combined analysis of outputs from the above technologies in multiomics analysis now holds significant promise to take these observations further. Indeed, the UKKW 2025 programme includes basic science sessions on computational strategies to understand kidney omics, multiomics in kidney disease research and translating strategies to regulate gene expression into novel kidney disease therapies.
In addition, UK laboratories continue to make notable strides using advances in other new technologies such as improved imaging techniques, the use of human-based disease models including the generation of kidney organoids (e.g., see Figure 2), and advanced gene therapy protocols. Using these methods in combination with established and developing technologies and models at leading UK kidney research centres, we are poised to learn much more about the molecular mechanisms intrinsic to the pathogenesis and progression of kidney disease, providing more effective means of predicting and treating kidney disease.
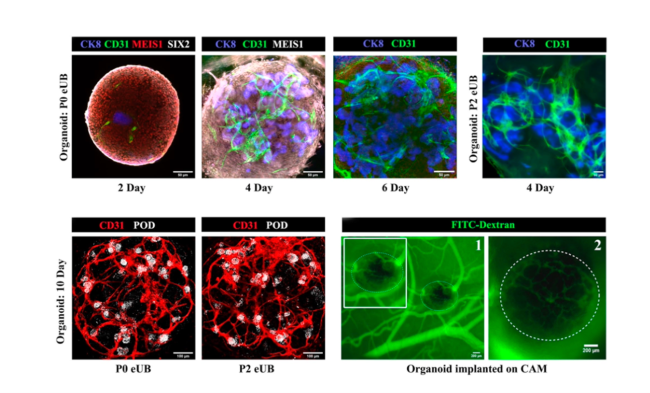
Figure 2. Small blood vessel formation in mouse embryonic stem cell organoids
Adapted from Palakkan et al. (2022) Production of kidney organoids arranged around single ureteric bud trees, and containing endogenous blood vessels, solely from embryonic stem cells. Scientific Reports 12:12573. doi.org/10.1038/s41598-022-16768-1. Licensed under CC-BY-4.0.
Enquiries to join the UKKA-LSRC
Please send initial enquiries to ukka@ukkidney.org
